You can probably tell that some foods are acidic because of how they taste (oranges, for example).
In this science project, we’ll use the juice from a colorful vegetable to indicate the pH level of different liquids and find out just how acidic or basic they are!
Red Cabbage pH Test Science Project
Learn how to use red cabbage to find out if a liquid is an acid or base.
Part 1: Make pH Test Strips
This part of the project requires chopping and cooking. Make sure you have an adult to help before you begin!
What You Need:
- half a red cabbage
- saucepan
- colander/strainer
- bowl
- rimmed baking sheet
- paper towels
- filter paper or coffee filters
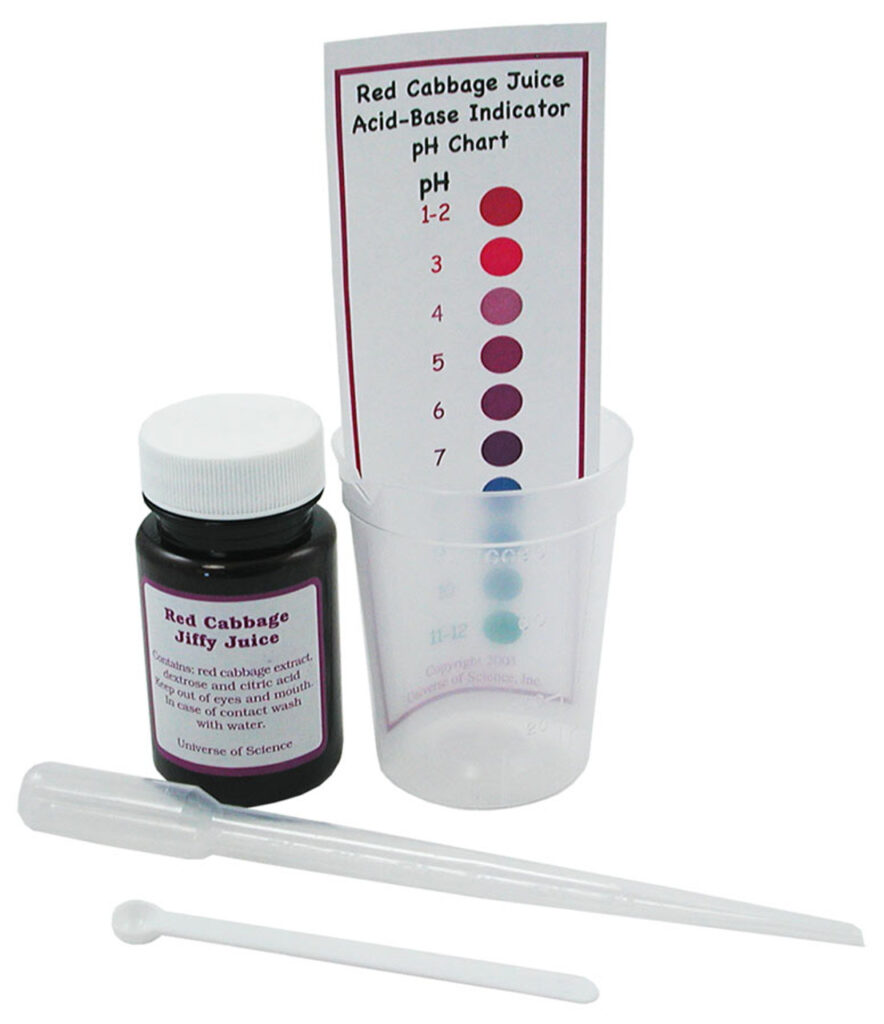
Buy this all-in-one convenient kit to test the pH levels of things!
Red Cabbage Indicator Jiffy Juice Kit
What You Do:
1. Ask an adult to chop the cabbage into small pieces. Put the pieces in the saucepan and cover with water.
2. With an adult’s help, heat the pan on the stove until the water begins to boil, then turn the heat to low and let the cabbage simmer for 20 minutes. Stir occasionally.
3. Let the cooked cabbage cool slightly, then have an adult pour the contents of the pot through the strainer, collecting the purplish cabbage liquid in a bowl.
4. Discard the cooked cabbage. Make sure to clean up any drips as you go because the cabbage liquid can stain.
5. Once the liquid is cool enough to touch, place the paper rectangles into the bowl and stir them around for a moment, then allow them to soak for about five minutes or until they have turned blue or purple.
6. Remove each paper and place it on a cooling rack to dry (put a rimmed baking sheet lined with paper towels under the rack to catch drips). Let the papers dry completely.
7. Once dry, cut the rectangles into strips about 1/2″ wide. Use these test strips for Part 2 of the experiment.





Part 2: Test Liquids for pH
Now for the exciting part of the project—see how different substances change the color of the test strips depending on how acidic or alkaline (basic) they are!
What You Need:
- test tubes and stand (or small jars)
- liquids from around your house to test (see list below)
- red cabbage test strips from above
- liquid substances to test*
- worksheet to track results
What You Do:
1. Pour a small amount of each liquid into a separate test tube.
2. Dip one test strip into each test tube and place it on a paper towel next to that tube. (Use a wooden skewer to pull the strip out if it isn’t long enough to grab with your fingers.)
3. Watch the test strips and note any changes in color on the paper.
4. Keep track of your results with this worksheet. You can even tape the test strips into the spaces once they’re dry. Note that the colors will lighten as they dry, so make sure to write down your results before the strips dry!



*Substances to Test (choose at least 6):
- Lemon juice
- Apple juice
- Cola or another soda
- Coffee or tea
- Milk
- Vinegar
- Baking soda (mix 1 teaspoon with 1 tablespoon of water)
- Dish soap or laundry detergent (mix 1 teaspoon with 1 tablespoon of water)
- Eggs (whisk the yolks and whites together)
- Antacid, such as Tums (crush a tablet with the back of a spoon and dissolve in 1 tablespoon of water)
What Happened:
The pigment that gives red cabbage its color is called anthocyanin. It is also the pigment found in leaves that turn red or purple in the fall. Anthocyanin is a good indicator of acids and bases, as you saw from the changing colors in this experiment. When added to a base, the purplish pigment turns green or yellow and when added to an acid, it changes to pink or red. In something that is neutral (neither an acid nor a base), the paper will remain the same color (or maybe turn a little blue).
Learn more about acids and bases and the pH scale in this Science Lesson.





