Energy in science may be electrical, mechanical, chemical, thermal, or nuclear.
Regardless of source, it’s measured by the work done.
Energy Science Lesson
You wouldn’t be using your computer if it weren’t for energy in many different forms!
The clack of fingers hitting the keyboard is what you hear when energy reaches your ears in sound waves.
The light from your computer monitor is energy. Electric energy from a power cord or battery makes your computer work. Your body is ‘powered’ by chemical energy in the food you eat. Your hands can feel heat energy coming from the warm monitor or bottom of the laptop. That’s a lot of energy!
But what are all these ‘energy’ forms and where do they come from? Keep reading to find out.
What is Energy?
Energy is usually defined as ‘the ability or capacity to do work.’
Work is the transfer of energy, usually defined as force applied over distance or force x distance.
Energy is measured in joules (1 newton of force applied over 1 meter distance) or foot-pounds (1 pound of force applied over 1 foot of distance).
Power is the rate of doing work or transforming energy from one form to another. Power is measured in watts (1 joule per second) or horsepower (550 foot-pounds per second). So a 60 watt light bulb converts 60 joules of electrical energy per second into light and heat energy. If you lift a box you are using energy from your body to do work.
The different forms of energy can be classed as either potential or kinetic. Potential energy is being stored, ready to do work. If a pencil is resting on the table, it has potential energy. If it falls from the table, that potential energy has been changed into kinetic energy, the energy of motion (with a boost from the kinetic energy of whatever gave it a push).
When the pencil hits the floor, some of its kinetic energy disperses. Eventually all of the pencil’s kinetic energy is transferred to the floor and it stops rolling.
Once it’s settled on the floor, it no longer has potential or kinetic energy. If you come along and move the pencil, your potential energy has been turned to kinetic energy, not the pencil’s!
This transfer of energy from one form to another without changing the total amount is called the conservation of energy. This ties into the first law of thermodynamics, which states that energy cannot be created or destroyed – it can only change form.
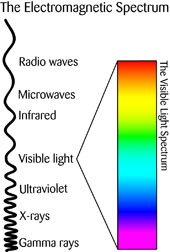
You are probably already familiar with energy called electromagnetic radiation, even if you’ve never heard the term before.
Visible light, x-rays, microwaves, radio waves, and the ultraviolet radiation that gives you a sunburn are all different types of electromagnetic (EM) radiation.
So what is EM radiation? Basically it’s a stream of tiny electrically-charged particles called photons, which travel in waves. They actually move in a special type of wave, called a transverse wave: one that doesn’t need a medium like air or wire to travel through.
This means that EM radiation can travel through the vacuum of space. All electromagnetic waves can travel at the same speed, the speed of light, which is about 186,000 miles per second! However, they only travel at maximum speed through a vacuum; things like water and air slow them down.
Transverse waves have oscillations (up and down or side to side movement) that are at right angles to the direction their energy is traveling. Since the electromagnetic spectrum is also made up of photons, it can act as either a stream of particles or as a wave. EM radiation is made of electrical fields and magnetic fields together.
Each type of EM radiation has different wavelengths and frequencies.
Frequency is the number of waves in a given time. The shorter the wave, the higher the frequency.
And the higher the frequency, the higher the amount of energy in the wave.
Wavelength refers to the distance between each complete wave cycle (e.g., two peaks right next to each other).
The sun is the source of much of the energy on this planet. Unlike the earth, the sun is not a solid; instead, it is a huge ball of gas, composed mainly of hydrogen.
Every second, the tiny nuclei (plural of nucleus) of tons of atoms fuse together to form molecules. Huge amounts of energy are released in the process. This energy is in the form of electromagnetic radiation.
Heat
Heat energy is often transferred by infrared electromagnetic radiation.
It is at a wavelength invisible to our eyes, but our skin can sense it. Heat energy can only be kinetic, since it is energy of moving particles.
The increased energy from the transfer makes molecules speed up. As they move faster, they bump into each other and spread out. Enough heat can break the bonds that hold molecules together as a solid, so they become a liquid. Add more heat and the liquid will become a gas.
Heat moves from hot temperatures to cold temperatures. It keeps moving until all the molecules around it are the same temperature (somewhere in between the original temperatures that were mixed).
This evened-out state is called thermal equilibrium. If you give a cup of oil and a cup of water an equal amount of heat, the oil will get hotter because it has a different thermal capacity – its molecules move faster.
Temperature is the measure of how hot or cold something is, based on how fast or slow its molecules are moving.
Two commonly used temperature scales are Celsius (C) and Fahrenheit (F). The freezing point of water is 0°on the Celsius scale and 32°on the Fahrenheit scale; its boiling point (when it turns into vapor) is 100°C and 212°F.
Light
Light is electromagnetic radiation in the middle of the spectrum. Its wavelengths are medium-sized, the only wavelengths that our eyes can detect. All of the colors of the rainbow are part of the visible light set of the electromagnetic spectrum. Red-colored light has the longest wavelength (just short of infrared) and violet-colored light has the shortest wavelength (just longer than ultraviolet radiation).
The sun and other hot sources produce incandescent light, which is light energy converted from heat. Fireflies, glow light sticks, and fluorescent bulbs convert other kinds of energy to light without using much (or any) heat.
Find out more about light energy in the Light & Optics web article.
Sound
Sound travels in longitudinal waves, which requires a medium, such as air, in order to travel. These are also compressional waves, formed when air is pushed away and then clumped together with empty spaces in between. Use a slinky to demonstrate how these waves work. Have someone hold one end of the slinky, and you hold the other. Spread apart so that the slinky is stretched out to about half its length. Now push your end of the slinky straight out toward the other person. The coils of the slinky will also push forward in bunches as the ‘wave’ ripples down the length of the slinky. When these waves go through your ear and are processed in your brain, they are converted to sound that you can hear.
Chemical
Chemical energy is potential energy stored in the chemical bonds that join atoms together. It can be converted to electrical, heat, or other energy through chemical reactions that break the bonds.
Food is a source of chemical energy. Our bodies store the potential energy until we need it. For example, when you sit down at the computer, your body converts some of chemical energy to another form, enabling you to move. Other common sources of chemical energy are gasoline and batteries.
Mechanical
Mechanical energy is associated with the movement or potential movement of an object. Springs and rubber bands have elastic potential energy; when they are stretched out they have the potential to shoot across the room when released.
There is also gravitational potential energy, the energy something has because of its position above the ground.
For instance, when you are holding a ball, it has potential energy from the force of Earth’s gravity pulling on it.
If you release it, the potential energy will be converted to kinetic energy as it falls.
The closer the ball gets to the ground, the more kinetic energy and the less potential energy it has.
Nuclear
Nuclear energy comes from fission, the splitting of atoms, or fusion, the joining of atoms.
Nuclear power plants use fission; the sun releases energy through fusion. One kind of uranium, U-235, is ‘unstable.’
When a stray neuron comes along, the unstable U-235 atom absorbs the neuron and then breaks apart into two atoms and more loose neurons.
A lot of energy is released in the process. In nuclear power plants, this is used to produce power: fission is induced, which releases heat energy, which causes steam, which turns the power plant’s turbines, which powers its generators, which provides electrical power to the area.
Right now a hot topic is whether we should be using more renewable energy sources than nonrenewable ones.
Sources of renewable energy are ones that we use without using up them. Some examples are the sun (in sunny climates, solar panels can capture its energy), wind (we can use its energy with windmills), and water, an element and compound, which powers hydroelectric dams.
Nonrenewable sources of energy, such as oil and coal, could eventually be used up; they aren’t being continually replaced the way that renewable sources are.
Noteworthy Scientist: Marie Curie (1867-1934)
Marie Curie, a famous scientist of the 20th century, was born in Poland with the name Maria Sklodowska. Her parents were both teachers and although her mother died when she was 10, her father was very influential in her education. Marie graduated from high school with highest honors but was suffering from depression, so her father sent her to spend a year on her cousins’ farm. Poland at this time was controlled by Russia, and the Polish people were allowed only a limited amount of education.
Marie and her sister Bronya studied at an illegal “floating university,” with night classes whose location changed frequently. The sisters agreed to help put each other through school in Paris, where women were free to go to universities. Marie worked as a governess for several years to put Bronya through medical school.
Marie taught herself basic chemistry during this time, as well as taught some Polish peasant children to read (even though it was against the law). Then her father got a better job and was able to finish paying for Bronya’s schooling, so Marie was able to save her money and go to Paris herself.
She registered at the famous Sorbonne university in 1891, officially changing her first name to Marie but keeping her Polish last name. In the beginning, Marie lived in an attic and at times had to wear all the clothing she owned just to keep warm. She worked hard and got her physics masters degree in three years, a math degree a year later, and was awarded a physics scholarship!
While looking for a laboratory where she could conduct research, she met Pierre Curie. He let her share his lab, the start of working side by side in scientific research for the rest of their lives. They were married a year later, in 1895.

Eventually Pierre’s father (a widower) moved in to help care for the Curies’ young daughters, Irene and Eve, while their parents were working in the lab.
Marie encouraged Pierre to finish writing his thesis and get his doctorate. He was proud of her own interest in science; she became the first woman in France to get a doctorate in science. Marie did her doctoral research on radiation, following up on Becquerel’s work with uranium and radiation.
He had discovered that uranium emits energy (radiation) without first absorbing energy from another source. Marie use an electrometer that Pierre and his brother had invented for measuring low electrical currents.
She proved that radioactivity is a property of uranium; the energy actually comes from the atoms that uranium is made of. Pierre shelved his own investigation of crystals and helped Marie conduct her research.
They discovered radium and polonium, which are radioactive elements in the uranium ore pitchblende. They worked in a shed because they couldn’t afford good laboratory conditions, although eventually others noticed their research and provided financial support for a better lab.
Marie rightly believed that radiation could be used for medical purposes, like killing cancer and diseased cells. She became the first woman to win a Nobel prize when she, Pierre, and Becquerel were awarded the prize for physics in 1903.
Just three years later, Pierre died when he fell and was crushed by a wagon. Both of them had been suffering health problems, although they refused to believe that it was from working with radioactive materials.
Marie continued to work hard after Pierre’s death. She founded the Radium Institute for research.
She took on Pierre’s professorship, becoming the Sorbonne’s first woman teacher. She also taught science once a week at her oldest daughter’s co-op school!
Marie won a second Nobel prize in 1911 (this time in chemistry, for her work isolating radium), the first person ever to win two Nobel prizes.
During the first World War, Marie and her daughter Irene trained others on the medical uses of radiation. After Marie died in 1934, Irene and her husband continued to research and received the Nobel prize in 1935 for discovering artificial radioactivity.
Energy Science Project
Make a Thermometer
Temperature is the measure of how hot or cold something is; the temperature changes based on the amount of heat energy something absorbs or loses, causing its internal energy to increase or decrease.
How does a thermometer measure temperature? Make your own to find out!
All you need is a glass bottle with a narrow neck, rubbing alcohol, red or blue food coloring, a straight plastic drinking straw, and some clay. (You can also use a 150 or 250ml flask, one-hole rubber stopper, and glass tubing.)
First, add a few drops of food coloring to half a cup of rubbing alcohol and pour the liquid into the bottle.
Then set the straw straight up in the bottle, with one end about half an inch from from the bottom of the bottle and the other end sticking out the top.
Use clay to make a stopper in the top of the bottle around the straw, holding it firmly in place.
When you do the experiment, be careful not to move the straw up or down, as that can affect the amount of liquid in the straw.
Place your thermometer in a bowl of cold water. What happens to the liquid in the straw?
Next place the thermometer in a bowl of hot water. What happens? The higher temperature makes the liquid in the straw rise, because the alcohol expands.
(You might want to use a permanent marker on the outside of the bottle to show where the level of the alcohol is at room temperature, at a cold temperature, and at a high temperature. Based on your findings, do you think alcohol expands much with standard household amounts of heat? Alcohol expands more with small temperature changes than water does. You can experiment with other liquids to see if they are as sensitive to temperature change.)
Although your homemade thermometer cannot accurately measure whether water is 60 °F, for instance, it does indicate whether a temperature is higher or lower than the temperature of the room.
(It needs to be calibrated to measure to degrees.)
Learn more about energy conversion from different power sources with this Energy Conversion Kit!





