Compounds and mixtures are sometimes used as synonyms by laypersons. While the terms do overlap, they’re technically not the same. Compounds are two or more elements chemically combined in definite proportions by weight. Mixtures are two or more substances not chemically combined. Read on for a more thorough explanation of the properties of compounds and mixtures.
The Basics – Molecules, Mixtures, & Compounds
The universe is made up of atoms, the tiny building blocks of matter.
This computer screen is made of atoms, as are your eyes, the rest of your body, and the floor underneath you!
In the last 110 years, scientists have discovered that atoms themselves consist of smaller particles. An atom has a central nucleus made up of protons and neutrons, surrounded by electrons.
These parts are so minute that scientists can’t see them. They’re “visible” only by the behavior of the atom. In case you’re confused, electron microscopes allow scientists to “see” highly magnified images of some atoms, but not the smaller particles that form the atom. 
Elements are the simplest substances in nature that cannot be broken down into smaller parts by normal chemical means. They contain only atoms of the same type, ones that have identical chemical properties. There are at least 90 naturally-occurring elements, plus man-made ones. If you look at a periodic table, you’ll see the names of each of these elements and some of their properties (such as mass, or how heavy the atom is).
Molecules & Compounds
When atoms from different elements are joined together in groups, they form molecules. The atoms in molecules bind together chemically, which means that the atoms cannot be separated again by physical means, such as filtration. The molecule has different properties from the elements from which is was made. A water molecule is not three separate atoms, two hydrogen (H) and one oxygen (O), but it is actually a unique H2O molecule.
Like elements that are formed of atoms of the same sort, compounds are formed of molecules of the same sort. The elements can be combined into about 2 million different compounds!
Did you know that eggshells are made up of a calcium carbonate compound? And citric acid, which is found in oranges and other citrus fruit, is a compound of carbon, hydrogen, and oxygen atoms. Your kids might find it helpful to do a science research project finding other common compounds around your house. Make hypotheses and do research using a chemistry reference book, web site, or text book to find out the answers.
Carbon is the most common element found in compounds. A compound containing carbon is called an organic compound.
Mixtures – A Bit of This and That
All matter can be classified into two categories: pure substances and mixtures.
A pure substance consists of a single element or compound. Iron is formed only of iron (Fe) atoms; table salt is formed only by sodium chloride (NaCl) molecules.
A mixture, however, is made up of different compounds and/or elements. When salt is added to water to make saltwater, it becomes a mixture. The salt and water molecules do not combine to form new molecules, but only “mix” together while still retaining their identities. Air is also a mixture, containing just the right amounts of nitrogen, oxygen, and other gases for life on Earth. 
Mixtures vary by composition. Homogenous (evenly-distributed) mixtures of two or more chemicals are called solutions. Salt water is a solution, Italian dressing is not (because the ingredients do not dissolve completely).
Mixtures of metals are called alloys (bronze is an alloy of copper and tin)
The substances that make up a mixture can be separated by physical means because they have different physical properties (such as different melting points) and are not chemically bonded.
A mixture can be separated into its parts in a variety of ways, including decantation (letting the sand in a mixture of water and sand settle, and then draining off the water, for example), filtering, and evaporation.
You can use a kitchen funnel and coffee filter for filtration, and either use sunlight or low heat for evaporation. Try out these methods on a saltwater and a sand and water mixture to see how they work and compare the results.
Evaporation will work for both saltwater and sand and water solutions, but filtration will not work for saltwater.
Can you think of other examples where a separating method will work for one mixture and not another? Another one to try is lemon juice, a mixture of water and citric acid; what do you think happens when it is boiled? The water evaporates and eventually leaves nothing but citric acid crystals.
A Chemical By Any Other Name…
The chemical formula for a molecule provides us with its atomic number. That is, it tells us the elements contained within and in what proportion.
For example, do you know how many atoms are in a molecule of AlSO4 (aluminum sulfate)? There is one aluminum (Al), one sulfur (S), and four oxygen (O). The answer is six atoms.
What is the formula for ammonium chloride? Since it contains one nitrogen (N), four hydrogen (H), and one chlorine (Cl), the chemical formula is NH4Cl. To familiarize your kids with the periodic table and the abbreviations for elements, make a game of answering questions like this. Figure out the number of atoms for CaOH2, FeCl2, K3FeCN6, and the chemical formulas for a compound with two sodium, one calcium, and three oxygen atoms and a compound with one iron and three chlorine atoms.
Have you ever wondered what to call a chemical compound, such as CaOH2? There are a few general rules that can help you. Start with the name of the first atom, Ca. That’s the element calcium. Then, take the next atom and replace the ending with “ide.” Oxygen (O) becomes “oxide”.
As with many rules, though, there are exceptions! In this case, the first part of the name for the last atom, hydrogen (H), is tacked on so that the correct chemical name is calcium hydroxide. Here’s one your kids should be able to figure out with just a periodic table: what is the chemical name for NaCl?
Prefixes, the part added to the beginning of a word, can help you figure out names and formulas, too. Mono means one, di means two. So a compound with two of the same molecule (represented by a subscript 2) would have “di” before that molecule name. What would you call CO2? How many oxygen atoms are in carbon monoxide? What is the chemical formula for dicarbon dihydride?
A mole is a measure of chemical substances. There are 602,300 million trillion (6.023 x 1023) atoms or molecules in one mole. A mole of any two elements with have a different mass, but the same number of particles. If you look up gold (Au) on a periodic table, you’ll see it has an atomic mass of 107.9. To measure out one mole, you should weigh out 107.9 grams of gold. The atomic mass of sodium (Na), on the other hand, is 23.0 and so one mole is 23 grams of sodium. Both gold and sodium have the same number of particles in one mole, though: 6.023 x 1023.
Taking It Further – Kinetic Theory & Change
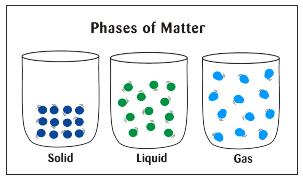
The kinetic theory of matter states that atoms and molecules are in constant motion and that the higher the temperature around them, the greater their speed will be.
In other words, increased heat energy will make atoms and molecules move faster This ties in to the states or phases of matter. In the solid phase, atoms and molecules are close together and vibrate in random directions. In the liquid phase, they move around and collide with each other. Then, in the gas phase, they move around much faster and spread out or expand. Energy is necessary for a phase change, often in the form of heat energy.
To show your kids how this works, do an experiment. Try making tea, using a mug of hot water and one of cold water to compare it to. Based on what was said in the previous paragraph about kinetic theory and molecule movement, will the hot or the cold water work best to mix the tea molecules?
The hot water molecules collide much more rapidly with the tea, mixing the molecules up faster. You might want to try this with other substances as well: food coloring, salt, and sugar, for example. Does boiling water work better than hot tap water for mixing the substances, or are they same? Point out to your young kids the steam coming from boiling water – this is the result of water molecules reaching boiling point and changing from liquid to gas.
When a solid reaches its melting point, it becomes a liquid. We see this when solid ice melts into liquid water. Then, when when the liquid is heated enough, it reaches boiling point and changes from liquid to gas phase. The melting and boiling points vary, depending on the properties of the elements that form the molecules. Water has a low melting point (32 °F), but many elements have much higher ones. Iron’s melting point is 2777 °F!
Chemical change affects the type of atoms in a molecule; physical change, on the other hand, does not change the atomic makeup of a molecule. When you take a piece of paper and hold the end to a flame, the result – burned paper – is a chemical change. The paper cannot become “unburned.” When you get a plain piece of paper wet, though, the change is physical. You can dry out the paper, often without leaving much damage – the water will evaporate. See if your kids can identify each of these changes as chemical (non-reversible) or physical (reversible): a nail getting rusty, juice freezing into a popsicle, wood burning in a fire, and sugar being mixed with water.
Bonds join different atoms or molecules together. Atoms have “shells” of electrons around them, which attract other atoms – when the outer shell has an incomplete number of electrons, it will attract another incomplete atom. A covalent bond occurs when atoms share electrons. Water (H2O) has very strong covalent bonds between hydrogen and oxygen atoms. A compound with covalent bonds will typically not conduct electricity when dissolved in water. (100% pure water does not conduct electricity.)
Ionic bonds typically form between metal and nonmetal compounds. A cation is an atom that has lost an electron and has a positive charge; an anion is an atom that has gained an electron and has a negative charge. When an ionic compound is dissolved in water, the water will conduct electricity.
In a reaction, the original bonds between atoms break down and the atoms form new bonds. Energy is used to break the old bonds and energy (usually in the form of heat) is given off or absorbed as the new bonds are formed.
Exothermic reactions produce heat, whereas endothermic reactions take heat in. Next time you bake a cake, consider this. The cake dough is not really a cake, but when it’s heated in the oven, an endothermic chemical reaction occurs and new bonds are formed, creating the compound of flour, sugar, butter, eggs, salt, and everything else that makes the cake.
Oxidation reactions occur when a chemical substance loses electrons to another substance. Combustion is an example of this. Gasoline contains carbon and hydrogen, which “oxidize” to produce water (H2O) and carbon dioxide (CO2) when it burns. Corrosion is another oxidation reaction, occurring when metal is in contact with oxygen. The reaction causes a compound, called an oxide, to form on the surface of the metal. Rust (iron oxide) is a common example of this.
In a reduction reaction, one chemical substance gains electrons from another substance. An example of this is plant photosynthesis: the plant cells use energy from the sun to turn water and carbon dioxide into glucose and oxygen.





