Salt molecules are made of sodium ions and chloride ions.
An ion either has a positive OR a negative charge based on whether it has gained or lost an electron. Gaining an electron equates to the atom having a negative charge and losing an electron gives the atom a positive charge.
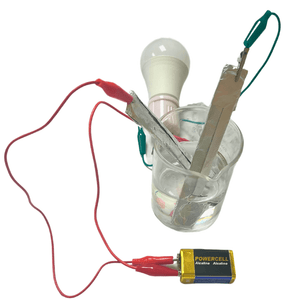
When you put salt in water, the water molecules pull the sodium and chlorine ions apart so they are floating freely, increasing the conductivity. These ions are what carry electricity through the water with an electric current. In short, saltwater (water + sodium chloride) acts as an electrolyte to transfer the electrical energy (current) through the water. While this can be done on a large scale, let's try a small-scale fun science project to see how it works! A project like this would make a great science fair project for elementary or middle school.
What You Need to Build a Saltwater Circuit:
- Glass cup or beaker
- Alligator clips
- Distilled Water
- Insulated copper wire
- Salt (table salt)
- 9-volt battery
- Aluminum foil
- A 3.7-volt lightbulb in a socket (or buzzer)
- Tongue depressors (or popsicle sticks)
Build a Saltwater Circuit in 8 Easy Steps
Circuit Kits to Take Learning to the Next Level





Science Lesson:
The lightbulb lit up because the sodium and chlorine ions conducted the electricity (an electrical current) from one electrode to the other.
The negative electrode is the anode and the positive electrode is the cathode. The electrons naturally flow from the negative anode toward the positive cathode because the electrons are negatively charged. The flow of electrons through that wire is electricity.
This completed the simple circuit, causing the lightbulb to illuminate.
Try adding more sodium chloride (salt) and see if the lightbulb illuminates brighter. Use a buzzer instead of a lightbulb and see if more or less salt in the water makes the buzzer ring louder or softer.